5968-11-6, F.W. 124.00, Sodium Carbonate, Monohydrate, Granular, Reagent, ACS - 39G888|S1230-2.5KG76 - Grainger

Scheme 3. Reagents and conditions: a) H2O, Na2CO3, rt, 1 min; b) CH3CN,... | Download Scientific Diagram

Phase Equilibria of the NaOH–NaBO2–Na2CO3–H2O System at 30 °C, 60 °C, and 100 °C | Journal of Chemical & Engineering Data
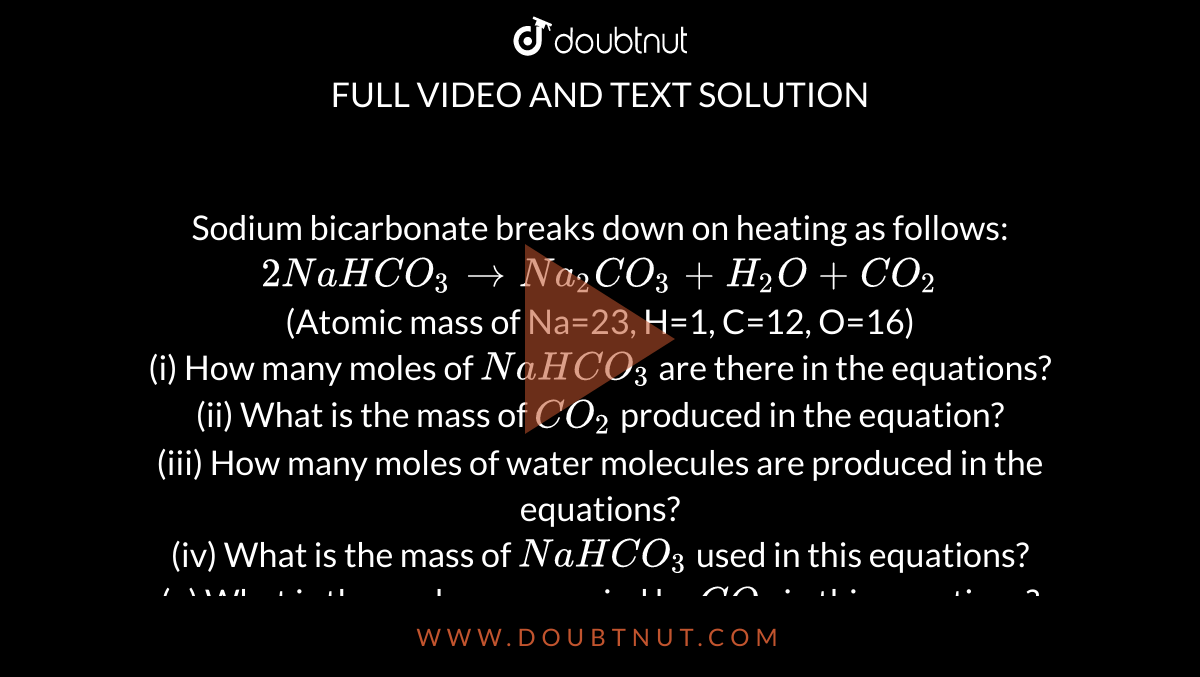
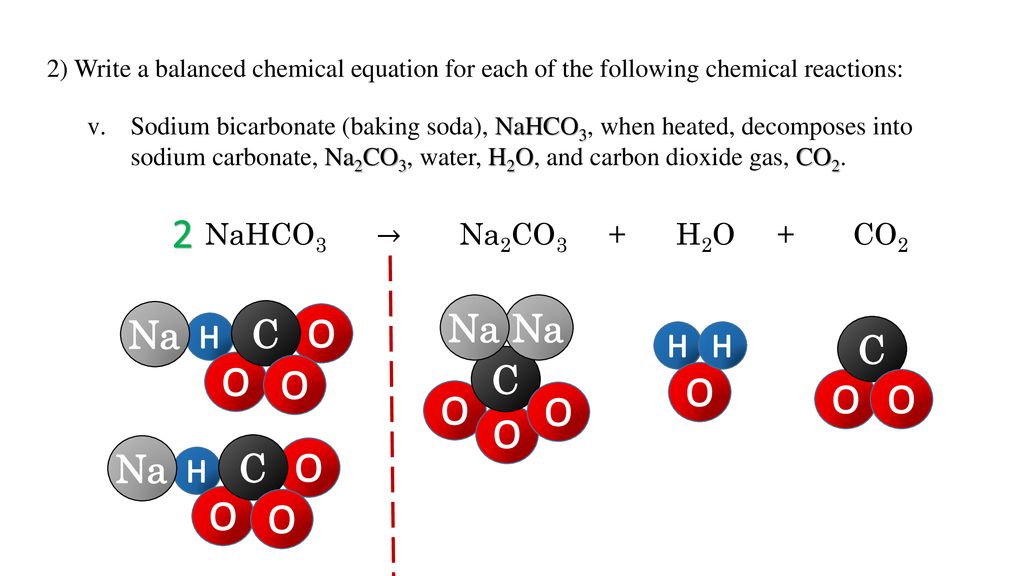
Sodium bicarbonate breaks down on heating as follows: 2NaHCO(3) to Na(2)CO(3)+H(2)O+CO(2) (Atomic mass of Na=23, H=1, C=12, O=16) (i) How many moles of NaHCO(3) are there in the equations? (ii) What is