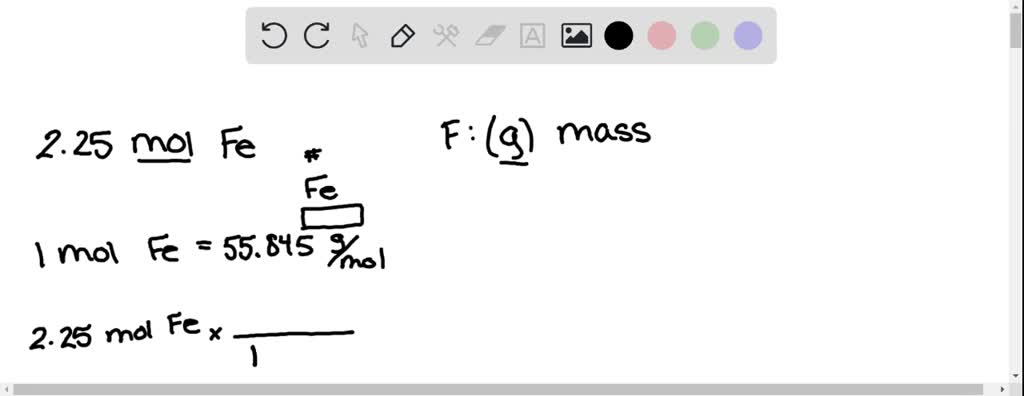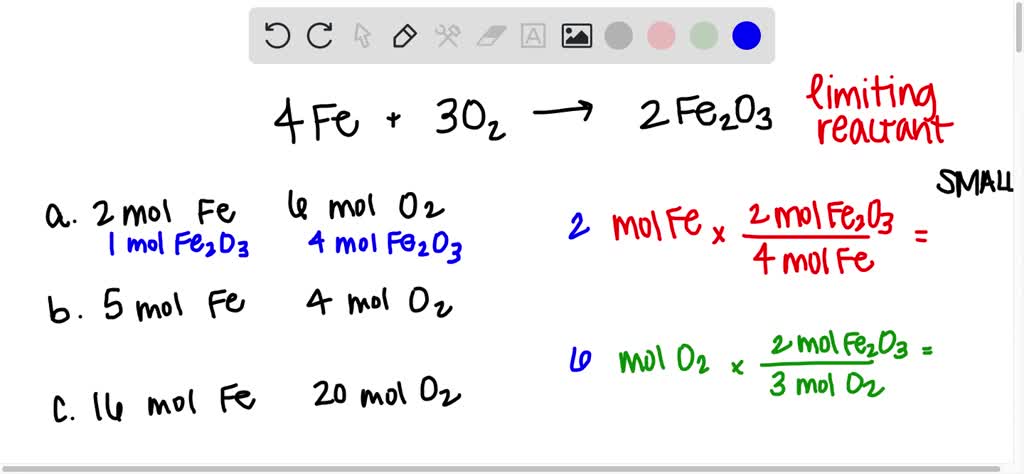
SOLVED:Iron and oxygen react to form iron(III) oxide. 4 Fe(s)+3 O2(g) ⟶2 Fe2 O3(s) Determine the limiting reactant in each of the following mixtures of reactants: a. 2.0 moles of Fe and

Iron standard solution traceable to SRM from NIST Fe(NO₃)₃ in HNO₃ 0.5 mol/l 1000 mg/l Fe Certipur® | Sigma-Aldrich

Jericho Rayel Timbol on Twitter: "Now let us to moles to mass ratio. Consider the reaction A → B Mass A can be converted to Mass B given that the molar masses
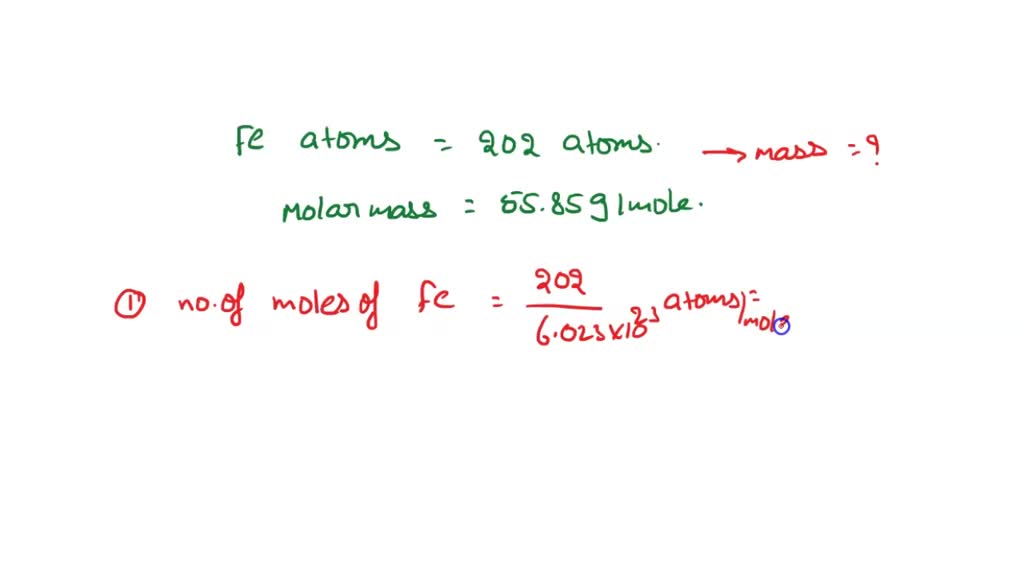
SOLVED: Question 3 of 97 Submit Calculate the mass, in grams, of 202 atoms of iron, Fe (1 mol of Fe has a mass of 55.85 g). 2 3 5 6 8 X10O