Draw resonance formulas for the azide ion, and for the nitronium ion. Decide which resonance formula is the best description of each ion. | Homework.Study.com

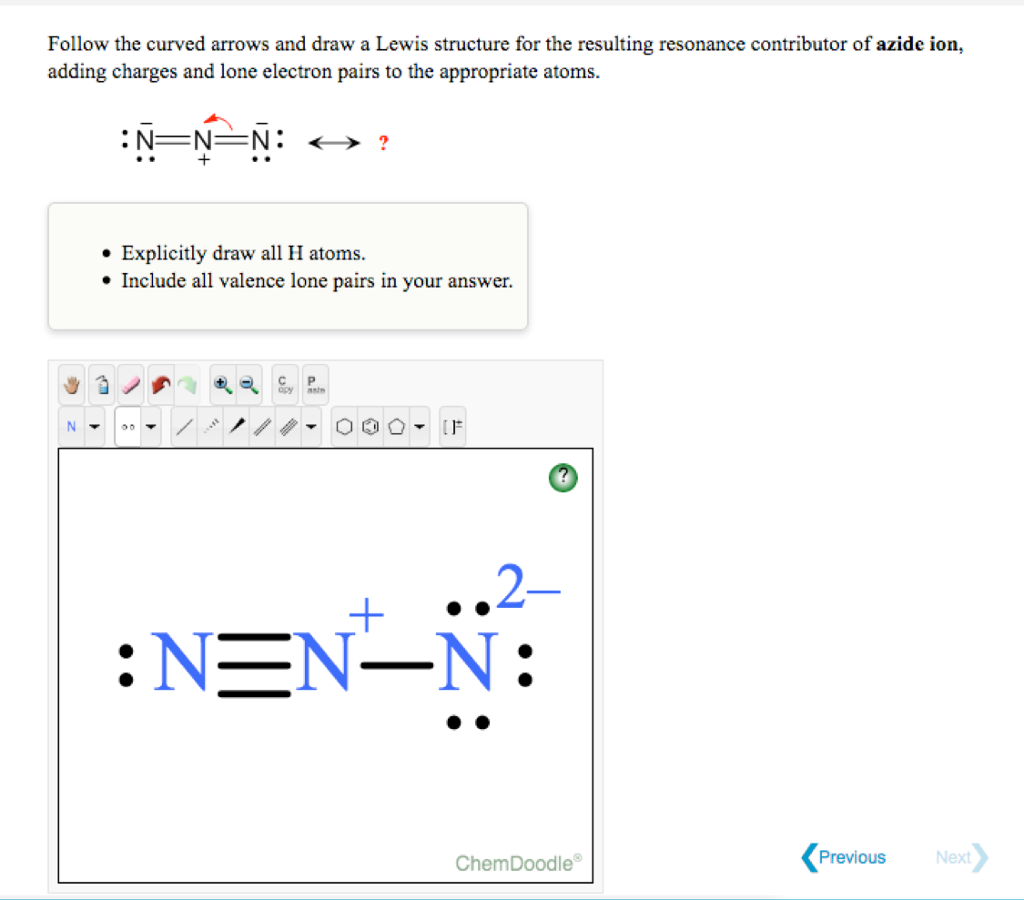
The azide ion, n−3, is a symmetrical ion, all of whose contributing structures have formal charges. - Brainly.com

Draw and explain the Lewis structure for the azide ion, showing all its possible resonance structures. Give the name of the molecular geometry and the hybridization around the central atom. | Homework.Study.com
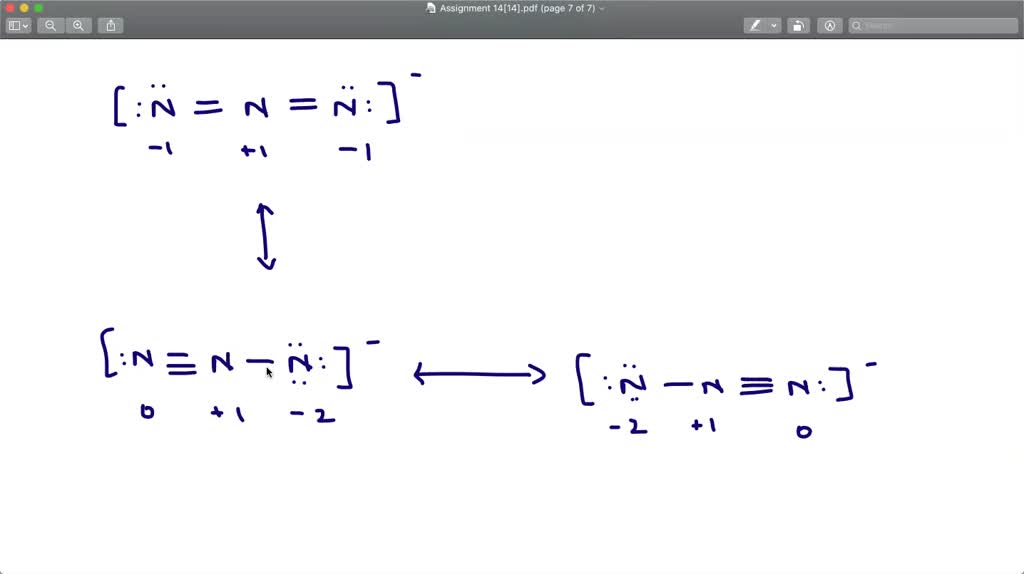
SOLVED:The azide ion, N3^-, is a symmetrical ion and all of its contributing resonance structures have formal charges. Draw three important contributing structures for this ion.

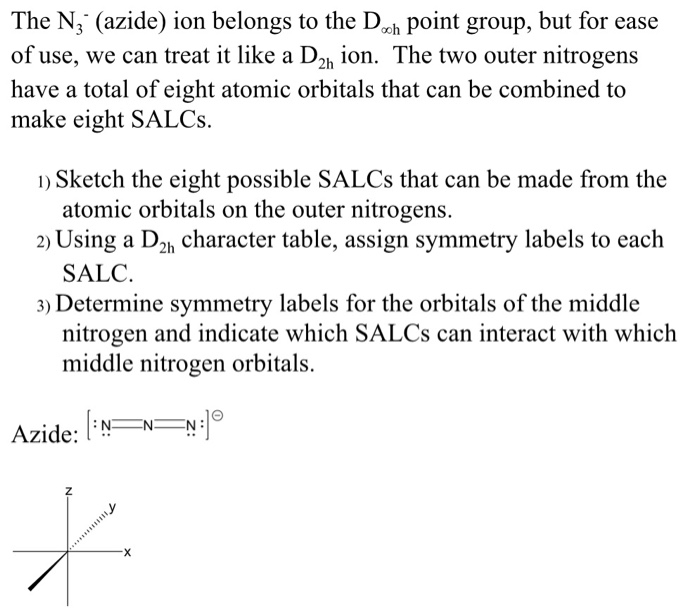
The azide ion (N_3) is a linear triatomic molecule. Determine the bond order and comment on how it compares to the Lewis Dot diagram for this molecule. | Homework.Study.com

Draw and explain the Lewis structure for the azide ion, showing all its possible resonance structures. Give the name of the molecular geometry and the hybridization around the central atom. | Homework.Study.com